ORDER NOW!
Contact your Dechra or Distributor Representative or call (866) 683-0660.
Dosing and administration of Zimeta
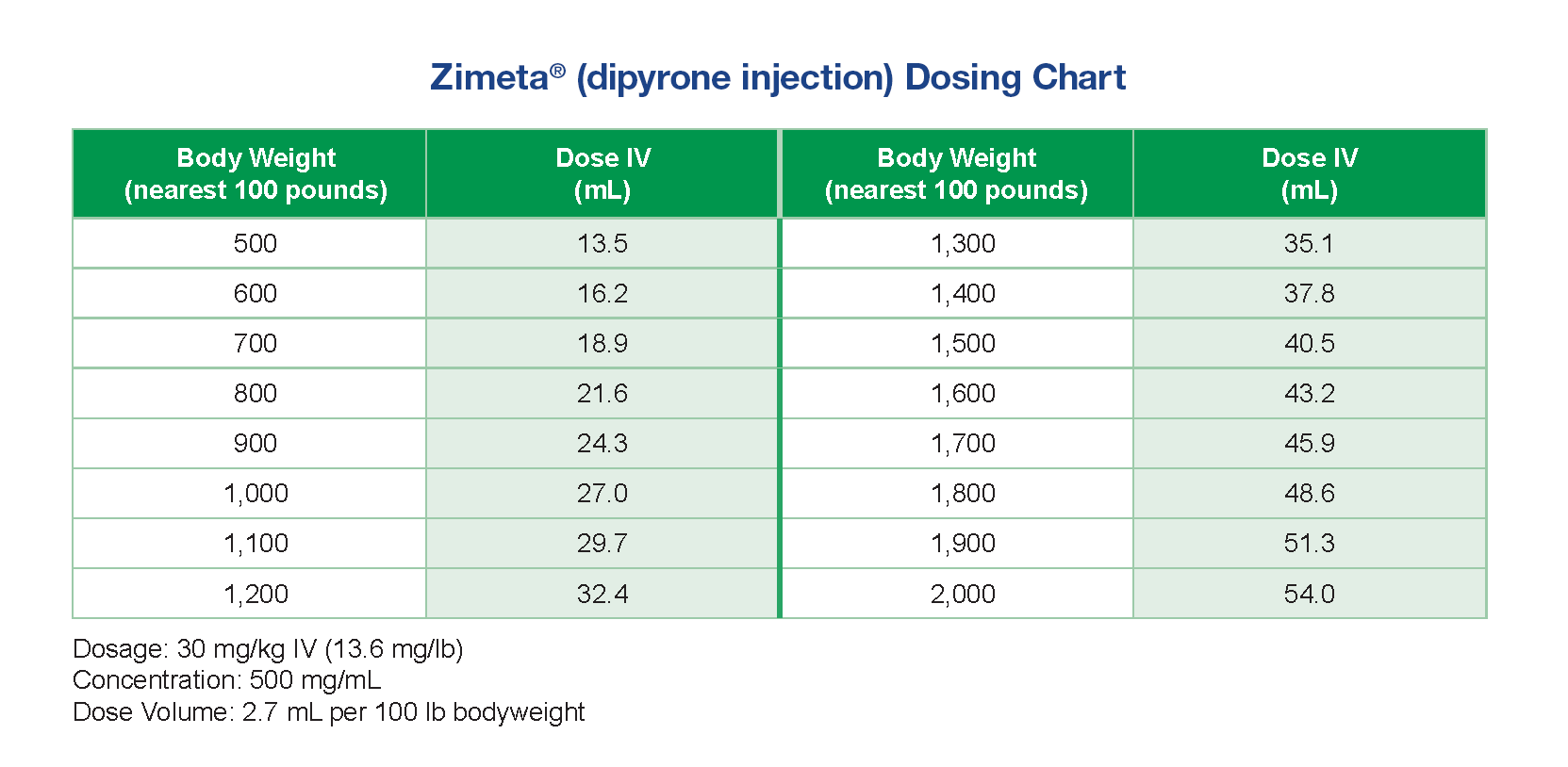
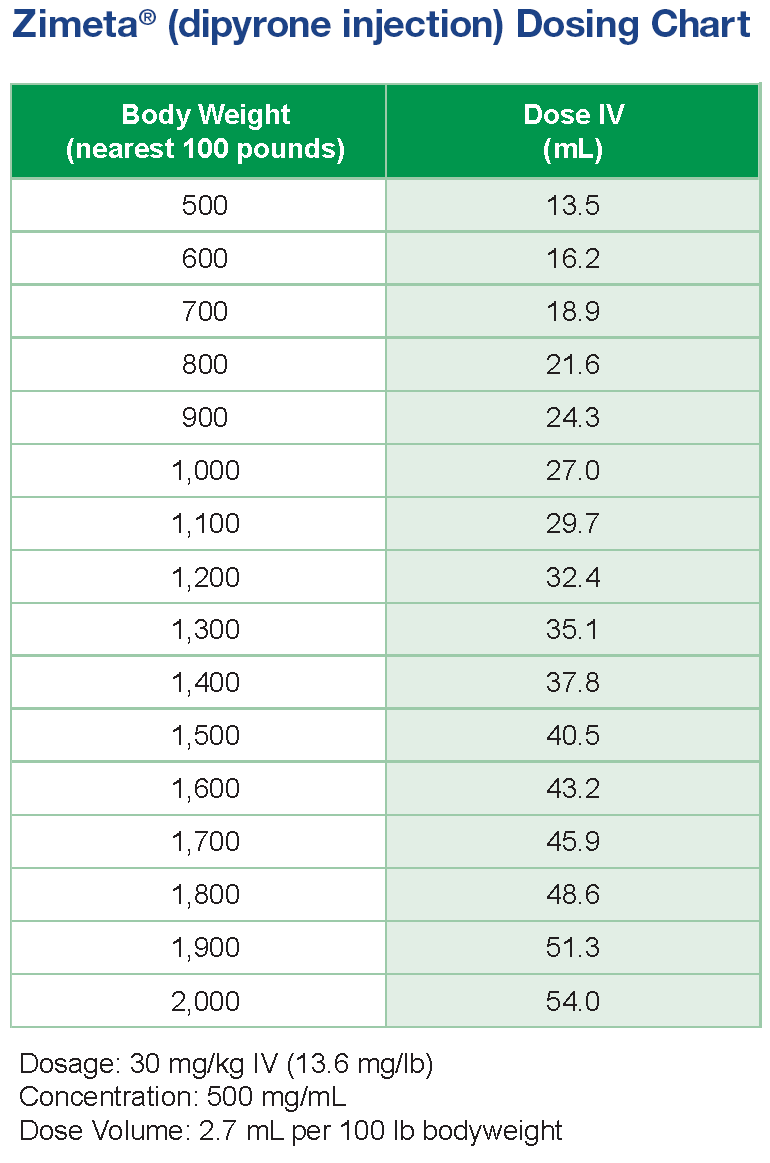
- Zimeta is provided as a 500 mg/mL injection in a 100 mL, multi-dose vial.1
- Administer Zimeta intravenously at 30 mg/kg every 12 hours as needed for up to three days.1
- Zimeta does not require refrigeration.
How to use Zimeta in competitive horses
Several organizations have provided guidance on the use of Zimeta and/or dipyrone, including the:
- United States Equestrian Federation (USEF)
- American Quarter Horse Association (AQHA)
- Fédération Equestre Internationale (FEI)
Of the non-steroidal anti-inflammatory drugs (NSAIDs) approved in the United States listed by the Fédération Equestre Internationale (FEI), dipyrone has the shortest established detection time in horses at 72 hours.2
The benefits of FDA-approved products
Unlike compounded preparations, FDA-approved drugs have been demonstrated to be safe and effective for their intended use and have been consistently manufactured to preserve the drug’s identity, strength, quality, and purity. On November 26, 2019, the U.S. Food and Drug Administration issued a letter reminding veterinarians of the benefits of using FDA-approved drugs and stated:
“The FDA strongly encourages use of an FDA-approved dipyrone animal drug product, when dipyrone is indicated, for use in horses for the control of pyrexia.”3
Zimeta is the only NSAID approved to control fever in horses
While NSAID is a broad functional classification, individual drugs within the class vary in FDA-approved indications, dose, dose frequency, and side effects. These variations should be considered by equine clinicians in addition to clinical signs and clinical experience when deciding the best course of treatment for their patients.
There are five FDA-approved NSAIDs for use in horses. Each belong to different chemical classes and have distinct clinical indications. The label indication for an FDA-approved drug indicates how the drug was studied and demonstrated to be safe and effective under the specific conditions of use.
Summary of NSAIDs Approved for Use in Horses for Intravenous Administration
| Drug Name | Chemical Class | Indication | IV Dose | IV Dose Frequency |
| Dipyrone1 | Pyrazolone | Control of pyrexia | 30 mg/kg (13.6 mg/lb) | Once or twice daily, at 12 hour intervals, for up to three days |
|---|---|---|---|---|
| Firocoxib4 | Cyclopropane5 | Control of pain and inflammation associated with osteoarthritis | 0.04 mg/lb (0.09 mg/kg) | Once a day up to 5 days |
| Flunixin meglumine6 | Carboxylic acid7 | Alleviation of inflammation and pain associated with musculoskeletal disorders
Recommended for the alleviation of visceral pain associated with colic | 0.5 mg/lb (1 mL/100 lbs) | Once a day for 5 days for musculoskeletal, repeat as needed |
| Ketoprofen8 | Propionic acid | Alleviation of inflammation and pain associated with musculoskeletal disorders | 1 mg/lb (1 mL/100 lbs) | Once a day up to 5 days |
| Phenylbutazone9 | Enolic acid, pyrazolidinedione | Relief of inflammatory conditions associated with the musculoskeletal system | 1 to 2 g/1,000 lbs (5 to 10 mL/1,000 lbs) | Once a day |
Sign up to receive future communications about Zimeta
For more information:
Contact your Dechra Representative
or call (866) 683-0660.
To place an order now:
Contact your Dechra Representative, Distributor Representative, or call (866) 683-0660.
Zimeta is indicated for the control of pyrexia in horses
As with all drugs, side effects may occur. Zimeta®(dipyrone injection) should not be given more frequently than every 12 hours due to the prolongation of prothrombin time (PT) and associated clinical signs of coagulopathy. For use in horses only. Do not use in horses with a hypersensitivity to dipyrone, horses intended for human consumption or any food producing animals, including lactating dairy animals. Not for use in humans, avoid direct contact with skin and keep out of reach of children. Care should be taken to avoid accidental self-injection and routine precautions should be used when handling and using loaded syringes as dipyrone can cause a deficiency in specific white blood cells in humans. Prior to use, horses should undergo a thorough history and physical examination by a veterinarian. Monitor for signs of abnormal bleeding and use caution in horses at risk for hemorrhage. Concurrent use with other NSAIDs, corticosteroids and drugs associated with kidney toxicity, should be avoided. Safety has not been evaluated in horses less than three years of age, horses used for breeding, or in pregnant or lactating mares. As a class, NSAIDs may be associated with gastrointestinal, kidney, and liver toxicity. The most common adverse reactions observed during clinical trials were elevated glucose conversion enzymes, decreased blood protein, gastric ulcers, inflamed or reddened lining of the right dorsal colon, and increased clotting times. Please see product insert for full prescribing information or visit www.dechra-us.com.
References
- Zimeta® (dipyrone injection) [package insert], Rev. 12/2020.
- Fédération Equestre Internationale. FEI List of Detection Times. July 13, 2018. Available from: https://inside.fei.org/system/files/FEI%20Detection%20Times%202018_0.pdf. [Access Date: March 1, 2021].
- U.S. Food and Drug Administration. Zimeta (dipyrone injection) – Veterinarians. Available at: https://www.fda.gov/animal-veterinary/product-safety-information/zimeta-dipyrone-injection-veterinarians. [Access Date: March 1, 2021].
- Equioxx® (firocoxib) [Full Prescribing Information], Merial, Inc. (Duluth, GA). Revised: 09/2016.
- National Center for Biotechnology Information. PubChem Database. Firocoxib, CID=208910. Accessed from: https://pubchem.ncbi.nlm.nih.gov/compound/208910 [Access Date: March 1, 2021].
- Banamine® (flunixin meglumine injection) [Full Prescribing Information], Intervet Inc., a subsidiary of Merck and Co., Inc. (Madison, NJ). Revised: 01/2017.
- National Center for Biotechnology Information. PubChem Database. Flunixin meglumine, CID=39212. Accessed from: https://pubchem.ncbi.nlm.nih.gov/compound/39212 [Access Date: March 1, 2021].
- Ketofen® (ketoprofen) [Full Prescribing Information], Zoetis Inc. (Kalamazoo, MI). Revised: 03/2013.
- Phenylbutazone 20% Injection [Full Prescribing Information], Aspen Veterinary Resources® Ltd. (Liberty, MO). Revised: 07/2015.
Zimeta® is a registered trademark of Kindred Biosciences, Inc. in the United States and/or other countries.
© 2021 Dechra Veterinary Products, LLC. All rights reserved.

